Determine Whether Each Compound Is Soluble or Insoluble in Water.
Consult the solubility table to answer the question. Soluble Insoluble Answer Bank Answer Bank LOH Al OH AgBr Hy CI Сасо NaF.

Solved Determine Whether Each Compound Is Soluble Or Chegg Com
Ions are listed as an exception.

. Determine whether each compound is soluble or insoluble in water. Pretty much all hydroxide except for those forms with alkali metals are insoluble so iron three hydroxide is insoluble while chlorides are soluble. Determine the limiting reactant.
The Results Are Shown In The Chart Below. Express your answer as a chemical formula. This option is true because the smaller the molecules the more hydrophilic they are.
The solubility rules shows which compounds are soluble in water. Soluble in water Insoluble in water ZnPO AgNO AlOH Answer Bank NAC. According to solubility rules all iodide anions are soluble but Ag.
Then we have led to chloride. Most chloride salts are soluble. -Ba NO_3_2 Determine the theoretical yield.
Theres a scalability rule that states all potassium or alkali metal salts are soluble and one that states all nitrates are soluble so this compound would be soluble. KBr AgBr BaSO4 Al2SO43 AlOH3. Notable exceptions are AgCl PbCl2 and Hg Cl.
Theres a rule that states all chloride salts are soluble but lead to is an exception. Determine whether each compound is soluble or insoluble in water. General Rules for Solubility of Ionic Compounds Salts in Water at 25C.
Determine whether each compound is soluble or insoluble in water. Determine whether each compound is soluble or insoluble in water. All nitrates are also soluble in waterThe hydroxide of copper is not soluble in water as well as the.
If It Does Dissolve No Mark Is Made. . KBr Al2SO43 Insoluble in water.
Determine the limiting reactant the theoretical yield and the percent yield. Identify the reactants and products. The first compound is potassium nitrate.
Click again to see term. -m BaSO4 315g Determine the percent yield. Choose the correct answer.
So this would be insoluble. Identify the precipitation reactions. 3 Smaller compounds are generally more soluble in water than larger compounds with similar structures.
Propane CH3CH2CH3- soluble in hexane. Soluble Insoluble Answer Bank Al OH3 AgBr CACO3 KBr NaOH HgCl. Several Solid Compounds Are Placed In Water To Determine If They Are Soluble.
Li Mostly soluble no exceptions Na Mostly soluble no exceptions K Mostly soluble no exceptions NH₄ Mostly soluble no exceptions NO₃ Mostly soluble no exceptions C₂H₃O₂ Mostly soluble no exceptions Cl Mostly soluble UNLESS paired with. A pin-connected truss is loaded and supported as shown. Solubility Rules A set of empirical rules used to determine whether an ionic compound is soluble.
Predict whether the following compounds are soluble or insoluble in water KNO3 CoBr2 AgNO3 AgBr BaSO4 NiCO3. Determine whether each compound is soluble or insoluble If the compound is from CHEM 131 at Montgomery College. Water H2O- insoluble in hexane.
A pin connected truss is loaded and supported as shown Each aluminum member has a cross sectional area of. -838 Each of the following compounds is soluble in water. According to the solubility rules all chlorides and bromides are soluble in water except those of silver and mercuryThis explains why most of the chlorides and bromides are designated as soluble.
Hydrogen chl ahat9181 ahat9181 08172020. HCl NaOH NaCl H2O. Solubility rules can help you identify precipitation reactions.
H2S- insoluble in hexane. Most nitrate NO3 salts are soluble. Therefore AgI is insoluble.
Except for a few exceptions Inc is not one of them was included is soluble and all sulfides are insoluble and there are a few exceptions. An X Indicates That The Compound Does Not Dissolve In Water. H2O2 NaIO NaI H2O O2.
Find an answer to your question Specify whether each compound is soluble in hexaneClassify the appropriate items to their respective category. The Top Row Shows The Cation In. Cu OH2 - Insoluble.
This statement is true compounds that form hydrogens bonds can interact with water. Tap again to see term. Assume a 475 ft and b 570 ft.
Most salts of Na K and NH4 are soluble. 2 Compounds that can form more hydrogen bonds are more soluble in water. Each aluminum member has a cross-sectional area of A 26 in2.

Solved Determine Whether Each Compound Is Soluble Or Chegg Com
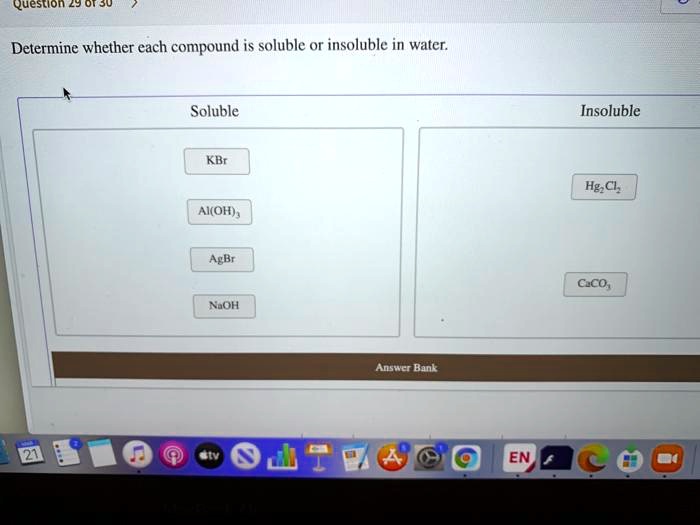
Solved Question Determine Whether Each Compound Is Soluble Or Insoluble In Water Soluble Insoluble Kbr Hg Cl Ai Oh 4 Agbr Ceco Noh Answcr Bank En

Solved Determine Whether Each Compound Is Soluble Or Chegg Com

Solved Determine Whether Each Compound Is Soluble Or Chegg Com
No comments for "Determine Whether Each Compound Is Soluble or Insoluble in Water."
Post a Comment